PRODUCT LITETATURE

PRODUCT LITETATURE
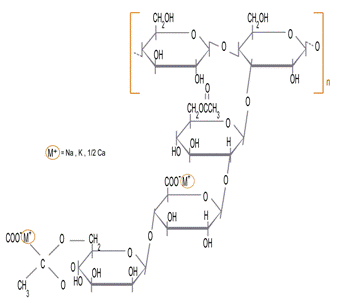
Xanthan has a particularly complicated molecular structure. however the backbone of xanthan is a β 1-4-D-glucose which is the same as cellulose. Every alternate glucose reside has a three sugar side chain consisting of two mannose residues with a glucuronic acid residue between them. The mannose reside nearest the main chain can carry a C6 acetyl group and the terminal mannose can carry a pyruvate group between C4 and C6. The acetylation and pyruvylation levels vary depending on fermentation conditions but typical values. Typically pyruvate residues can be found on 30-40% of the terminal mannose residues whereas 60-70% of the internal mannose residues may contain acetate groups. Recent work has looked at the properties of GM modified strains of xanthan gum that are either deficient in acetate groups, pyruvate groups or both.
Xanthan is produced in its native state as a twin stranded, right handed five fold helix. The stability of the helix is strongly affected by the ionic environment. Upon heating the xanthan helix goes through a transition to a disordered state and upon cooling it reverts to a helical structure. However it is believed that native xanthan exists in a form where chains are paired and once that has been lost and the xanthan molecules allowed to reorder the exact pairing cannot be retained and a partially crosslinked structure is formed as helices twist around various neighbours.
Xanthan Gum is a naturally occurring polysaccharide produced by fermentation process into a white to creamy powder. It is the most versatile hydrocolloid with excellent multiple functionalities as thickener, suspension and emulsification agent as well as stabilizer. Xanthan gum is odorless, nontoxic and readily hydrates in water as anionic polysaccharide solution suitable for both food and other industrial applications. The typical physical properties of xanthan gum are:
(1) High Viscosity: High solution viscosity at low concentration. A highly functional thickener.
(2) Pseudoplasticity: Solution viscosity decreases with increasing shear rate. Solution viscosity recovers with the cessation of mixing, ideally suitable for many industrial processes applications.
(3) Temperature Insensitivity: Solution viscosity is generally not affected over a wide range of temperature of -4 to 120 degrees C. in the presence of salt (for example: 0.1% NaCl).
(4) Salt Tolerance: Xanthan solution usually maintains its functions in the presence of salts such as NaCl even up to saturation level.
(5) Acid/Base Tolerance: Solution viscosity is generally stable over a wide range of pH from 2 to 12.
(6) Compatibility and Synergism: Compatible with acid, alkali, salts, preservatives and other hydrocolloids. Synergistic with locust bean gum, konjac gum and guar gum.
(7) Resistant to oxidation and enzyme hydrolysis. Xanthan gum is generally resistant to oxidation and enzyme hydrolysis.
(8) Suspension and Emulsification Effects: Capable of suspending particles and emulsifying oil in aqueous solution.
The above functionalities Xanthan Gum make it ideally suitable for use in the Food, Pharmaceutical, Cosmetics/Personal Care, Paper, Textile, Ceramics, Chemicals and Petroleum Drilling as well as many other applications

